Solid Biosciences Announces Positive Feedback From Type C Meeting With FDA for SGT-003 Gene Therapy for Duchenne Muscular Dystrophy (Globe Newswire)

Solid Biosciences Inc., which acquired UF startup AavantiBio, announced a positive regulatory update from its recent Type C meeting with the U.S. FDA that supports the continued advancement of SGT-003 as a potential treatment for Duchenne muscular dystrophy.
Oragenics Partners With DUCK FLATS Pharma To Support FDA IND Readiness and Clinical Trial Design for Concussion Program (Globe Newswire)

UF Innovate | Accelerate graduate Oragenics announced it has engaged DUCK FLATS Pharma as its U.S. Investigational New Drug (IND) readiness and regulatory execution partner to support FDA-facing preparation and clinical trial design as the Company advances its novel intranasal concussion therapy toward U.S.-based development.
TuHURA Biosciences Received FDA Orphan Drug Designation for IFx-2.0 for the Treatment of Stage IIB To Stage IV Cutaneous Melanoma (TuHURA Biosciences)
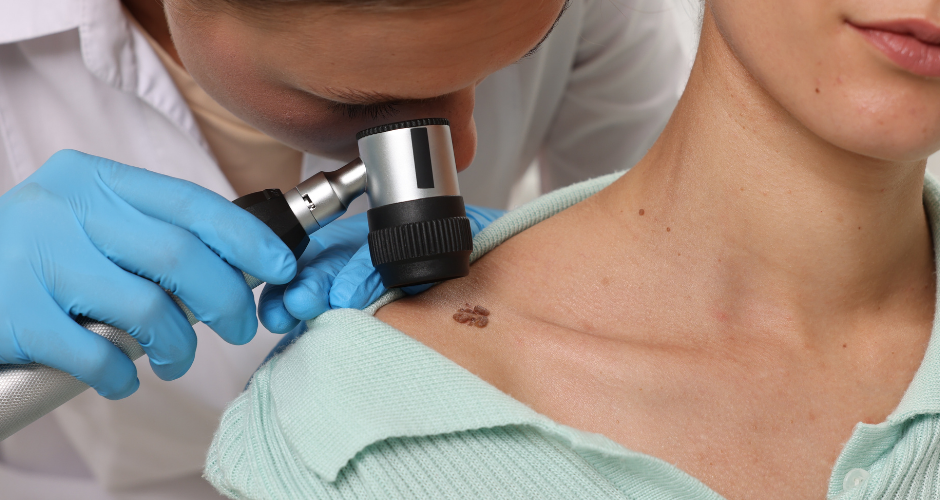
TuHURA Biosciences, formed by CohBar and Morphogenesis, a UF startup, announced that the U.S FDA has granted Orphan Drug Designation (ODD) to IFx-2.0 for the treatment of stage IIB to stage IV cutaneous melanoma.
Dr. Stephan Schmidt Named Chair of the Department of Pharmaceutics (UF Pharmacy)

UF researcher Stephan Schmidt, Ph.D., F.C.P., has been appointed chair of the Department of Pharmaceutics in the University of Florida College of Pharmacy.
Axogen Announces FDA Approval of Biologics License Application for AVANCE® (Globe Newswire)
UF startup Axogen, Inc., announced that the U.S. Food and Drug Administration has approved the Biologics License Application for AVANCE® (acellular nerve allograft-arwx).
Solid Biosciences Receives FDA Rare Pediatric Disease Designation for SGT-212 Dual Route of Administration Gene Therapy for Friedreich’s Ataxia (Globe Newswire)
Solid Biosciences Inc., which acquired UF startup AavantiBio, announced that it received Rare Pediatric Disease designation from the U.S. Food and Drug Administration (FDA) for SGT-212, the Company’s investigational gene therapy for Friedreich’s ataxia (FA).
NeXtGen Biologics Successfully Completes First FDA Audit With Zero 483 Observations (Globe Newswire)

UF Innovate | Accelerate resident client NeXtGen Biologics announced the successful completion of a two-day inspection by the U.S. Food and Drug Administration (FDA).
Myosin Therapeutics Receives FDA Fast Track Designation for MT-125 in Glioblastoma (PR Newswire)
UF startup Myosin Therapeutics announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to MT-125 for the treatment of glioblastoma (GBM).
Solid Biosciences Receives FDA Fast Track Designation for SGT-501 First-in-Class Gene Therapy for Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)
Solid Biosciences Inc., which acquired UF startup AavantiBio, announced that it received Fast Track designation from the U.S. Food and Drug Administration (FDA) for SGT-501, the Company’s novel, AAV-based investigational gene therapy for the treatment of catecholaminergic polymorphic ventricular tachycardia (CPVT).
Genascence Announces U.S. Food and Drug Administration Grants Regenerative Medicine Advanced Therapy Designation to GNSC-001 for Knee Osteoarthritis (OA)
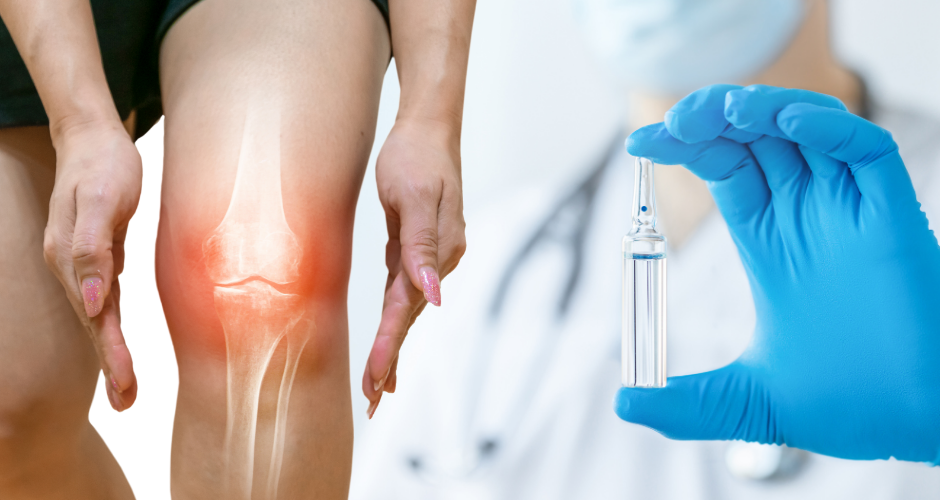
UF startup Genascence Corporation announced that the U.S. FDA has granted Regenerative Medicine Advanced Therapy (RMAT) designation to GNSC-001, a potential first-in-class gene therapy that targets interleukin-1 (IL-1) for the treatment of knee osteoarthritis (OA)
