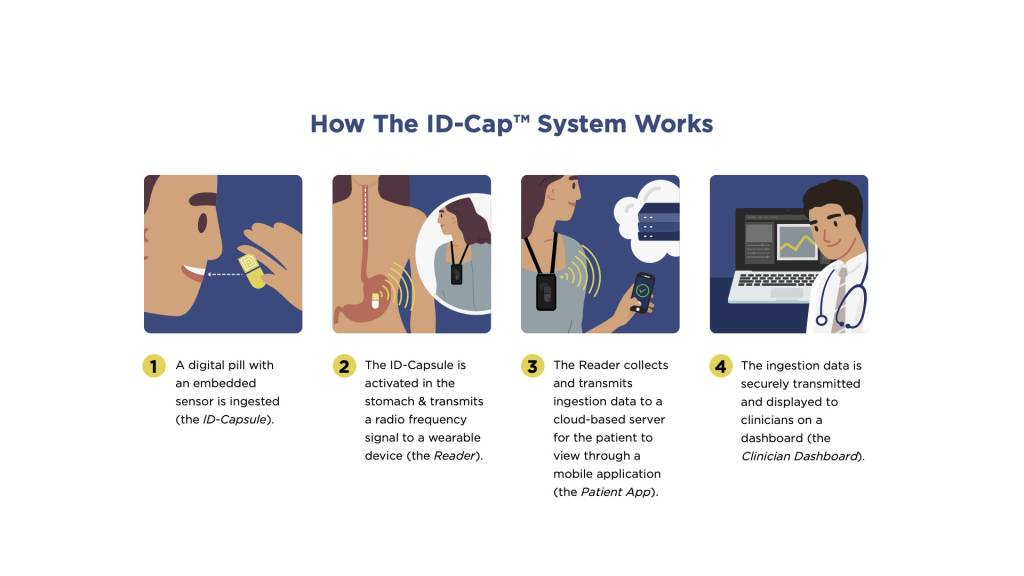
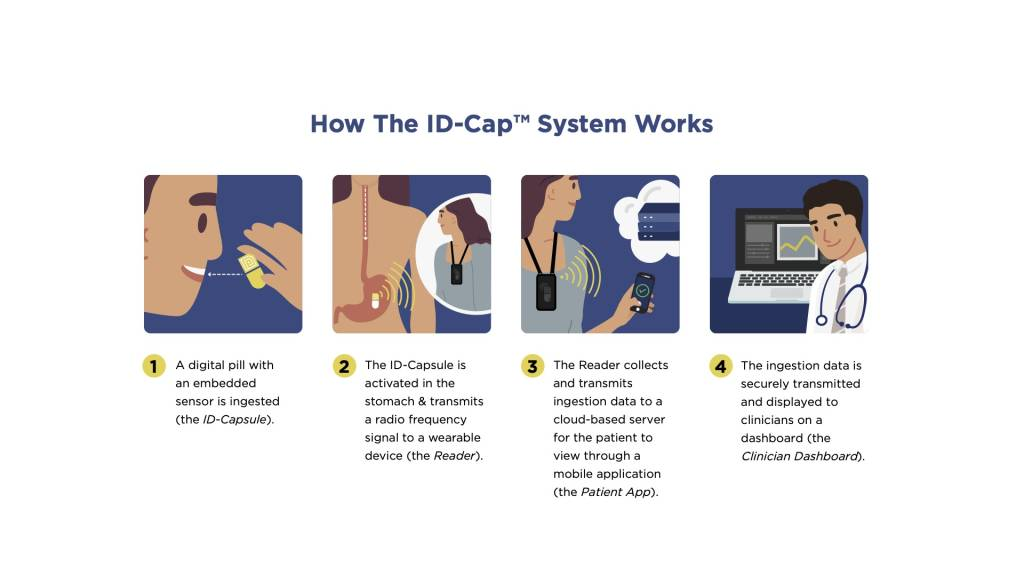
etectRx announces record-breaking use of the ID-Cap™ System, utilized in University of Colorado’s HIV treatment study
The Quantification of Tenofovir Alafenamide Adherence and Exposure in Adults living with HIV (QUANTI-TAF) study evaluated drug concentrations in dried blood spots (DBS), in tandem with medication adherence measured by the ID-Cap™ System. The ID-Cap™ System’s remote patient monitoring (RPM) capabilities successfully enabled the QUANTI-TAF study to produce one of the largest and longest digital pill studies in HIV treatment history.
A seamless, end-to-end solution, the ID-Cap™ is the only ingestible sensor solution which allowed the study’s researchers to monitor, track, trend, report, and provide real-time, accurate data through etectRx’s core technology, eBurst. When nonadherence was detected by the ingestible sensor technology, University of Colorado researchers were able to intervene in a timely fashion to send real-time dosing reminders to patients’ smartphone apps.
QUANTI-TAF began in August 2020, and the last follow-up concluded in May 2023. A total of 84 participants were enrolled in the study for up to 4 months each, resulting in 8,910 expected ingestions over 9,049 days monitored, and a cumulative adherence rate of 95%. The QUANTI-TAF study showed consistently high acceptability of the ID-Cap™ System over time, as participants used it for up to approximately 112 days (4 months).
etectRX is a resident client of UF Innovate | Accelerate at The Hub in Gainesville, Florida.
Read more about ID-Cap™ System Powers Historic HIV Digital Pill Study.