Oragenics Targets Projected $9 Billion Market by Advancing First and Only Clinical-Stage Concussion and Mild Traumatic Brain Injury Therapy (Globe Newswire)

UF Innovate | Accelerate graduate Oragenics, Inc. outlined its 2026 milestones as it advances ONP-002, a novel intranasal neurosteroid in clinical development for concussion and mild traumatic brain injury.
Solid Biosciences Announces Positive Feedback From Type C Meeting With FDA for SGT-003 Gene Therapy for Duchenne Muscular Dystrophy (Globe Newswire)

Solid Biosciences Inc., which acquired UF startup AavantiBio, announced a positive regulatory update from its recent Type C meeting with the U.S. FDA that supports the continued advancement of SGT-003 as a potential treatment for Duchenne muscular dystrophy.
Oragenics Partners With DUCK FLATS Pharma To Support FDA IND Readiness and Clinical Trial Design for Concussion Program (Globe Newswire)

UF Innovate | Accelerate graduate Oragenics announced it has engaged DUCK FLATS Pharma as its U.S. Investigational New Drug (IND) readiness and regulatory execution partner to support FDA-facing preparation and clinical trial design as the Company advances its novel intranasal concussion therapy toward U.S.-based development.
Amend Surgical Announces ISO 13485 Certification, Advancing Toward Launch of Amend Tissue Tape™ (PR Newswire)
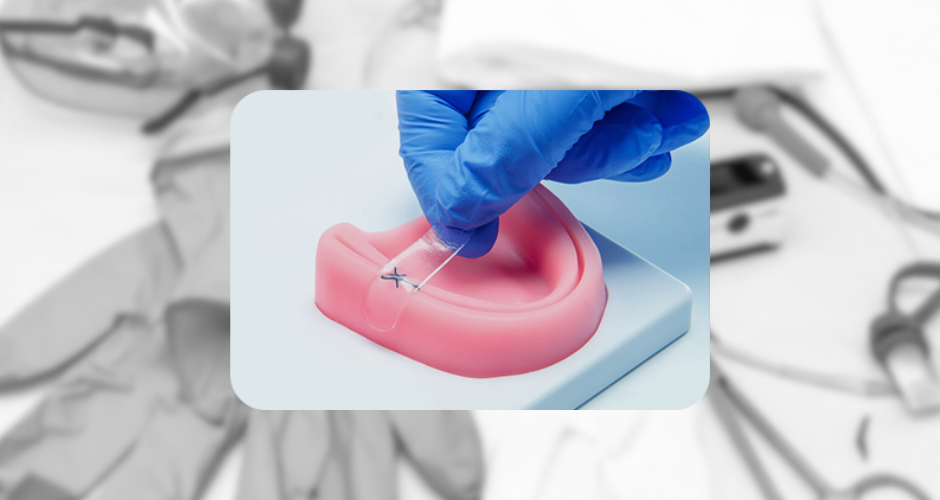
UF Innovate | Accelerate client Amend Surgical announced that it has achieved ISO 13485:2016 certification, the global standard for medical device quality management systems.
Mark Cuban Cost Plus Drugs and Alchem Laboratories Announce Alliance To Advance Essential Medicines (EIN Newswire)

UF Innovate client Alchem Laboratories announced a partnership with Mark Cuban Cost Plus Drugs to support the development and delivery of essential medicines.
Solid Biosciences Announces Duchenne Muscular Dystrophy Added to National Recommended Uniform Screening Panel by the U.S. Department of Health and Human Services (Bio Space)

Solid Biosciences Inc., which acquired UF startup AavantiBio, shared that the U.S. Department of Health and Human Services (HHS) officially added Duchenne muscular dystrophy (Duchenne) to the Recommended Uniform Screening Panel (RUSP), the list of conditions recommended for universal newborn screening across the United States.
TuHURA Biosciences Announces Its Release of Kintara’s Contingent Value Right (CVR) As Kintara’s REM-001 Meets Primary Safety Endpoint Achieving Contractual Milestone (PR Newswire))

TuHURA Biosciences, formed by CohBar and Morphogenesis, a UF startup, announced that Kintara’s REM-001 clinical trial of ten metastatic cutaneous breast cancer patients met its primary endpoint, demonstrating safety with signs of clinical efficacy following eight weeks of follow-up for such patients.
NeXtGen™ Biologics Secures Favorable Outcome in Inter-Partes Patent Review (BioSpace)

UF Innovate | Accelerate resident client NeXtGen Biologics reported that the U.S. Patent and Trademark Office declined to initiate an inter partes review of its wound care patents, leaving the intellectual property fully enforceable.
30 Under 30 – Energy & Green Tech (Forbes)

Forbes has announced its 30 Under 30 list for Energy & Green Tech, recognizing innovators shaping the future of sustainable technology. Among the honorees are Tamas Kereszy, Caroline Comeau, and Jason Gibson.
Solid Biosciences Receives FDA Rare Pediatric Disease Designation for SGT-212 Dual Route of Administration Gene Therapy for Friedreich’s Ataxia (Globe Newswire)
Solid Biosciences Inc., which acquired UF startup AavantiBio, announced that it received Rare Pediatric Disease designation from the U.S. Food and Drug Administration (FDA) for SGT-212, the Company’s investigational gene therapy for Friedreich’s ataxia (FA).
